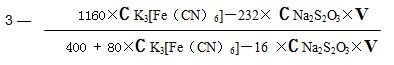Blue Tungsten Oxide Oxygen Index Testing Method

1.Principle
Blue tungsten oxide oxygen index testing is based on its solubleness in potassium hydroxide and potassium ferricyanide solution, potassium tungstic acid(K2WO4) is obtained. Overdosed potassium ferricyanide solution will react with potassium iodine in acidic solution, iodine is released. Titrate with sodium thiosulfate standard solution to calculate blue tungsten oxide oxygen index.
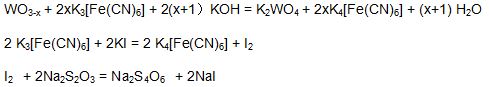
2.Agent
(1)Potassium hydroxide, analytical grade, 10g/100ml.
(2)Hydrochloric acid, analytical grade, 1+1.
(3)Potassium iodide, analytical grade, 10g/100ml.
(4)Zinc sulfate, analytical grade, 10g/100ml.
(5)Starch, analytical grade, 1g/100ml.
(6) Potassium bichromate titrant: Weigh 2.4515g and dry it under 140~150℃ and dissolve it in water, move into 1000ml volumetric flask, dilute to scale, mix them. Concentration is 0.05mol/L.
(7) Sodium thiosulfate standard solution: Weigh 12.5g sodium thiosulfate standard solution and dissolve in water, add 0.1g soda, mix, filter in 1000ml brown bottle, dilute with water, put it still for a week for standardization. Concentration is 0.05mol/L.
Standardization: Weigh 20ml, 0.05mol/L bichrome standard solution in 250ml triangular flask, add 20ml HCl, 20ml iodine potassium solution, mix, put it still for 5min, tilt with sodium thiosulfate solution until it turns light brown, add 1ml starch solution, keep tilting until blue color of solution disappears. Volume of sodium thiosulfate solution is V0ml, chemical reaction:
3 Na2S2O3 + K2Cr2O7 + 6HCl = 2 Cr(OH)SO4 + 4 NaCl + 3S + 2H2O + KCl + Na2SO4
Calculate sodium thiosulfate molar solution:
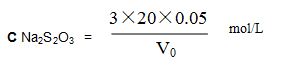
(8)Potassium ferricyanide standard solution: Weigh 66grams of analytical potassium ferricyanide standard solution in 250ml flask, add water and dissolve, move into 1000ml bottle, add water and dilute, mix, concentration is 0.02mol/L.
Standardization: Weigh 5ml potassium ferricyanide standard solution in 250ml triangular bottle, add 20ml HCl, 20ml iodine potassium solution, mix, put it still for 5min, tilt with sodium thiosulfate solution until it turns light brown, add 1ml starch solution, keep tilting until blue color of solution disappears. Volume of sodium thiosulfate solution is V1ml, calculate potassium ferricyanide standard solution molar concentration:
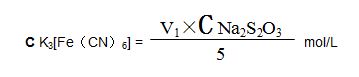
2.Analyze Method
Weigh 0.2grams sample in 250ml triangular bottle, add 5ml potassium ferricyanide standard solution , 15ml potassium hydroxide solution, blow and clean the side of bottle, heat and dissolve for 15min under 70℃ until sample completely dissolved, cool down to room temperature. Add 20ml hydrochloric acid, 20ml potassium iodide solution, mix them and put it still for 5 min, tilt with 0.05mol/L sodium thiosulfate solution until it turns light brown, add 1ml starch solution, keep tilting until blue color of solution disappears. Volume of sodium thiosulfate solution is Vml.
2.1Oxygen Index Calculating: